Imagine...
Imagine a future in which we didn’t need to test drugs on animals any more. And imagine that the doctor could check to see which form of medication was best for your body. The secret is a chip with thousands of electrodes and perhaps even built-in mini-microscopes.
So, imagine that you donate some skin cells or blood – and these can be reprogrammed to become heart cells, for instance. And then, thanks to cleverly designed structures, materials and molecules on the chip, these cells grow like they would in a real heart, complete with beating movements and contraction waves that propagate. Various “wells” (as in a cell culture plate) are applied on top of the chip and various types of medication are placed automatically in the wells (and hence on your heart cells). The electrodes and microscopes take readings of the condition of the cells and then, after all of the data has been processed, a decision can be taken about which medicine your heart cells respond best to.
Stressful for the heart
As part of the process to develop a new drug, major trials have to be conducted before any medicine can be licensed: the drug is tested on cell cultures, lab animals and – in the end – on humans. But many of these developmental medicines never make it to the market because they are too toxic for the heart.
And even those drugs that do reach the market can be stressful for many people’s hearts and even cause irreparable damage. Just think of those cancer patients who become so debilitated by their chemotherapy that ultimately they die, not of the cancer, but of a heart problem. And even if the outcome is not so drastic, it’s still important to keep the heart in good condition to ensure successful treatment and faster rehabilitation afterwards.
To eliminate this problem of cardiotoxicity completely, ever-larger and more expensive trials are required. Which also means that fewer new drugs are launched. The solution is twofold: allow tests on cardiotoxicity to run more efficiently and faster during the development phase of medication, and – if the drug is approved – conduct tests on each patient to see how their heart cells respond to a specific form of medication.
Animal tests or in vitro tests?
Animals are used to test new drugs, although this is kept to a minimum for ethical reasons. These tests are also expensive, time-consuming and often not representative for the human body.
In vitro tests (cells in petri dishes) are often used as a supplement. These tests are cheaper, require less time and are ethically more acceptable. But they are not ideal, because they are not always representative. Today, cell lines are used that don’t always grow or respond in the same way as the cells that are being investigated, such as heart cells.
Yet in vitro tests are definitely the way of the future if we can improve them in such a way that the cells in the petri dishes behave more like they do in the human body. This is an area in which chip technology can be useful.
The in vitro test of the future
Imec has been developing for some time a chip with electrodes on which cells can grow. The electrodes give small electrical pulses to the cells (e.g. for electroporation) or measure electrical signals from the cells (e.g. brain cells and heart cells).
Four years ago, work began on research for a doctorate (by Thomas Pauwelyn) in conjunction with KU Leuven and the Gasthuisberg University Hospital. The goal of this research was to further optimize the chip for in vitro testing of drug cardiotoxicity. The building blocks for just such a chip were tested to ultimately produce a platform with microfluidics, built-in mini-microscopes and separate ‘chambers’, each with around 1000 electrodes.
The electrodes are perfect for measuring electrical signals from heart cells. Traditionally, this is done with the patch-clamp method in which a thin pipet makes contact with the heart cell. However, this is a destructive method. There are also some newer systems, also based on electrodes, which measure the electrical signals from heart cells non-destructively. The electrode chip described here is unique because the electrical signals inside the cell are measured first by opening the cell membrane using electroporation (electrical stimulation of the cell using electrodes). Because this method of measuring is non-destructive, the heart cells can be measured intact for a longer period.
Imec’s “lens-free microscope” technology has also been tested in this context, since it is important to monitor the way the heart cells contract. Knowing this can provide an indication of their response to certain types of medication. We also get information about how representative the test is: do the cells grow in the same way as they would in the human body? Do they have a beating movement? Is the contraction wave also passed on to cells that are alongside each other?
The unedited images from the lens-free microscope were used in this first phase of the research. These unedited images were sufficient to check how much the cells change shape and contract and relax. So this way, the platform also includes a ‘sensor’ for monitoring the contraction of the heart cells.
And it is precisely this combination of electrical and contractile sensors that make this chip so valuable for screening cardiotoxicity in the development of new drugs. Some existing chips also measure both parameters, but in different areas on the chip (the electrical activity is measured in some cells and the contraction in others). The imec chip is the only one that measures both parameters on the same cells.
This is not just about fundamental research. The aim is also to have this chip used by pharmaceutical companies, specialists in hospitals, etc. Which is why it is important to produce a cheap (and disposable) chip. For this reason, researchers opted to use silicon technology –well-known from computer chips – because these chips can be produced in large quantities and are relatively cheap.
In another project – InforMed – the researchers focused on making this chip platform compatible with the standard testing methods used in the pharmaceutical industry. In this instance, cell culture plates were used with a standard number of wells or spaces into which cells, molecules, etc. can be placed. A unique form of packaging to accommodate these plates was created with Micronit Microtechnologies. The chip has 16 zones, each with 1000 electrodes. Each zone is connected with a microfluidic space or ‘chamber’ via little channels. The pipetted fluid (e.g. a medicine to be tested) is carried via these microchannels to one of the 16 zones in which, for example, heart cells are growing.
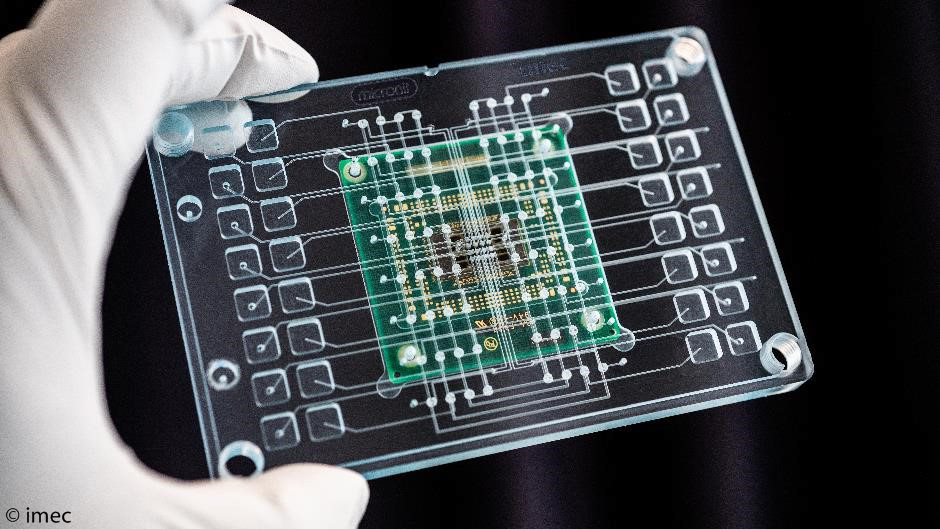
Printed circuit board with the multi-electrode chip that is connected with microwells in a cell culture plate. 16 different types of medication are then administered, which are taken along fine channels to the chips on which cells grow.
More research
More research is needed before this chip can be used for standard in vitro tests for developing drugs or for selecting the right medication for a patient. For example, multiple cell models will be tested on the chip, such as cells from sick and healthy patients, to see whether they respond differently to certain types of drugs. Processing all of the results from the measurements from the electrodes and mini-microscopes is also very difficult. Specific software for doing this is still being developed.
But this chip is certainly an important step toward ‘personalized medicine’ in which patients are treated in a targeted, well-adjusted manner. Researchers in a range of different disciplines – pharmacy, stem cell research, chip technology – are doing everything they can to make this happen.

“The innovative approach using cardiac organ-on-chip systems developed by imec opens up novel and exciting perspectives for drug development and disease modeling in cardiovascular translational research. The technology offers the prospect of high throughput introduction in clinical practice, thereby making it possible to combine novel biological insights in cardiovascular disease with targeted therapies under development.”
- Professor Dr Stefan Janssens, Cardiologist UZ Gasthuisberg
Want to know more?
- You can request the paper entitled ‘Reflective lens-free imaging on high-density silicon microelectrode arrays for monitoring and evaluation of in vitro cardiac contractility’ via our contact form.
- The ISSCC paper ‘A 16384-Electrode 1024-Channel Multimodal CMOS MEA for High-Throughput Intracellular Action Potential Measurements and Impedance Spectroscopy in Drug-Screening Applications’ is also available via our contact form.
- You can find more information about InforMed, funded by the ECSEL Joint-undertaking (ECSEL2014-2-662155 ), on the project website.

Dr Thomas Pauwelyn has studied at KU Leuven since 2008. He earned his BSc in Bioscience Engineering specializing in Catalytic Technologies in 2011 and a Master’s in Nanoscience and Nanotechnology with the Bioscience Engineering option in 2013. Thomas completed a PhD at KU Leuven and imec’s Life Science Technologies group in 2018. He received an IWT fellowship to carry out his PhD and has also successfully applied for a post-doctoral innovation Mandate Grant from VLAIO. Thomas’s research focuses on developing novel organ-on-chip systems for predictive toxicology and drug development.

Prof. Dr. Stefan Janssens obtained his medical degree in 1984 from the University of Leuven, Belgium, summa um laude, and finished his clinical cardiology fellowship at Gasthuisberg University Hospital, Leuven, Belgium. He subsequently obtained an international John E. Fogarty fellowship from the NIH (Bethesda, MD, USA) to continue his studies in cardiovascular medicine at Massachusetts General Hospital, Harvard University in Boston from 1989-1992 and was appointed Professor of Medicine in 2002 at KU Leuven and Chairman of the Department of Cardiovascular Diseases in 2010.
His clinical interest focuses on cardiac intensive care and his research is primarily directed towards translational studies on myocardial dysfunction and cardiac repair. The prime research in the laboratory focuses on different progenitor cell populations, on the Nitric Oxide (NO) / cyclicGMP signaling system, and on therapeutic interventions in patients with ischemic heart disease and reduced left ventricular function.
More about these topics:
Published on:
30 May 2018












